xenon tetrafluoride hybridization|Hybridization of XeF4: Hybridization of Xe in Xenon Tetrafluoride : Baguio Hello Guys! Today in this video, we are going to learn the hybridization of the XeF4 molecule. It is a chemical formula for Xenon Tetrafluoride. To understand the hybridization of the. Learn the rules of Gold Ball for LOTTO 649 & play for a chance to win two jackpots & other prizes. Learn about winning ticket numbers, draw rules & more.
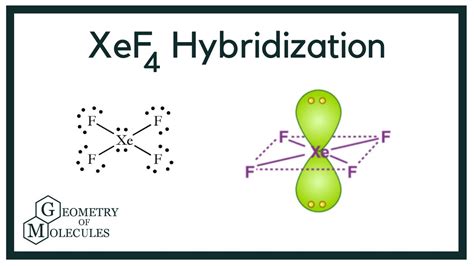
xenon tetrafluoride hybridization,What is the Hybridization of Xenon Tetrafluoride? In xenon tetrafluoride, the hybridization takes place in the central atom which is Xenon (Xe). If we look at the valence shell of Xe there are a total of six electrons in the 5p orbital and two electrons in . Hello Guys! Today in this video, we are going to learn the hybridization of the XeF4 molecule. It is a chemical formula for Xenon Tetrafluoride. To understand the hybridization of the. It is produced by the chemical reaction of xenon with fluorine, \(\ce{F2}\), according to the chemical equation: \[\ce{Xe + 2 F2 → XeF4} \nonumber \] The infrared .
The central Xenon atom’s orbitals are hybridized, which results in the formation of new hybridized orbitals. Xenon has six .
xenon tetrafluoride hybridizationXenon tetrafluoride is a chemical compound with chemical formula XeF 4.It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:. Xe + 2 F 2 → XeF 4. .The hybridization in xenon tetrafluoride occurs in the central atom, Xenon (Xe). When we look at Xe’s valence shell, we can see six ions in the 5p orbital and a pair of . I quickly take you through how to draw the Lewis Structure of XeF4 (Xenon TetraFluoride). I also go over hybridization, shape and bond angle.XeF4 Geometry and Hybridization. Xe is the central atom: There are 4×7 + 8 = 36 electrons and 8 are taken to make 4 covalent bonds. Each fluorine takes 3 lone pairs, so there are 36 – (8+4×6) = 4 electrons left which go . This video explains the determination of hybridisation of xenon tetrafluoride using group theory.Hybridization of XeF4: Hybridization of Xe in Xenon Tetrafluoride The hybridization of Xenon Tetrafluoride is determined using the valence bond theory, which takes into account the number of valence electrons and the number of bonds formed by the central atom. In the case of Xenon Tetrafluoride, there are four bonds and two lone pairs, resulting in a hybridization of sp3d. 3. Also, the process of hybridization is the development of the valence bond theory. For exploring this knowledge in advance, we will apply three kinds of hydrocarbon compounds to explain sp 3, sp 2, and sp hybridization. As we know, in the case of XeF 4 or xenon tetrafluoride, the hybridization of xeof₄ occurs in the central atom, which is . XeF4 Lewis Structure: Drawing easy steps,Hybridization,shape. May 10, 2022 by Mansi Sharma. [custom_reviewer] In the XeF4 lewis structure, xenon is a noble gas that has 8 valence electrons. Similarly, fluorine belongs to group 17 of the periodic table and has 7 valence electrons. To achieve octet stability 4 fluorine atoms will share their 1 .
Formula: F 4 Xe. Molecular weight: 207.287. CAS Registry Number: 13709-61-0. Information on this page: Phase change data. Gas phase ion energetics data. References. Notes. Data at other public NIST sites:
Other articles where xenon tetrafluoride is discussed: chemical bonding: Applying VSEPR theory to simple molecules: The XeF4 (xenon tetrafluoride) molecule is hypervalent with six electron pairs around the central xenon (Xe) atom. These pairs adopt an octahedral arrangement. Four of the pairs are bonding pairs, and two are lone pairs. According to . Hello Guys!Today in this video, we are going to learn the hybridization of the XeF4 molecule. It is a chemical formula for Xenon Tetrafluoride. To understand.

In the intriguing process of hybridization within Xenon Tetrafluoride, the central atom Xenon (Xe) is the protagonist. Observing Xenon's valence shell, we find six electrons in the 5p orbital and a pair in the 5s orbital. The 5th shell, interestingly, has vacant d and f orbitals. During XeF 4 's formation, the 5p orbital donates two electrons .

Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z .Lewis Dot of Xenon Tetrafluoride. Xe does not follow the octet rule. It actually bonds. It will hold more than 8 electrons. Xenon having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons. XeF 4 is d 2 sp 3 hybridized and contains 2 lone pair and 4 bonding pairs of .xenon tetrafluoride hybridization Hybridization of XeF4: Hybridization of Xe in Xenon TetrafluorideStep-by-Step Guide to Drawing the Lewis Structure of XeF4. 1. Count the total number of valence electrons. Xenon (Xe) is in Group 18 of the periodic table and has 8 valence electrons. Fluorine (F) is in Group 17 and has 7 valence electrons. Since we have four fluorine atoms, the total number of valence electrons is: 8 (Xe) + 4 * 7 (F) = 8 + 28 .xenon tetrafluoride. Formula: F 4 Xe; Molecular weight: 207.287; CAS Registry Number: 13709-61-0; Information on this page: Gas phase ion energetics data; References; Notes; Other data available: Phase change data; Data at other public NIST sites: Gas Phase Kinetics Database; Options: Switch to calorie-based units; This process is known as hybridization. If we need to learn about the hybridization of xenon difluoride, we have to look at the respective electronic configurations. Xe: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 Or, Xe: [Kr] 4d10 5s2 5p6. F: 1s2 2s2 2p5 Or, F: [He] 2s2 2p5. So, we can look at all the atomic orbitals of the .Xenon Tetrafluoride: Heat Capacity and Thermodynamic Functions from 5 to 350°K. Reconciliation of the Entropies from Molecular and Thermal Data. The Journal of Chemical Physics 1972 , 57 (8) , 3401-3408.What is the Hybridization of Xenon Tetrafluoride? In xenon tetrafluoride, mixing occurs with the central atom Xenon (Xe). If we look at the Xe valence shell there is a total of six electrons in the 5p orbital and two electrons in the 5s orbital. If we look at the 5th shell then there is a d orbital and an orbital where there are no electrons.Square planar structure of Xenon Tetrafluoride . XeF 4 is a planar molecule. Valence bond representation of XeF 4 may be explained if, two electrons from 5p orbitals are promoted to 5d orbital. One 5s, three 5p and two 5d atomic orbitals of xenon hybridize to give six sp 3 d 2 hybridized orbitals. The four singly occupied hybridized orbitals are used by four . Xenon tetrafluoride (XeF4) is a crystalline compound that is normally colorless or white. It is composed of xenon (a noble gas) and fluoride (a naturally occurring mineral). XeF4 can be used to detect and analyze trace metals that contaminate silicone rubber. XeF4 is a chemical compound made up of Xenon (Xe) and fluorine atoms.Xenon tetrafluoride (XeF. 4. ) – D. 4h. CONTROLS. Click the Symmetry Operations above to view them in 3D. XeF 4 belongs to the D 4h Point group and contains; One C 4 rotation axis, one C 2 rotation axis (equivalent to C 42 ), Four C 2 axes perpendicular to the C 4 axis. 4σ planes of symmetry,one σ h plane.The sulfur atom in sulfur hexafluoride, SF 6, exhibits sp3d2 hybridization. A molecule of sulfur hexafluoride has six bonding pairs of electrons connecting six fluorine atoms to a single sulfur atom. There are no lone pairs of electrons on the central atom. To bond six fluorine atoms, the 3 s orbital, the three 3 p orbitals, and two of the 3 d .
xenon tetrafluoride hybridization|Hybridization of XeF4: Hybridization of Xe in Xenon Tetrafluoride
PH0 · Xenon tetrafluoride
PH1 · Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis
PH2 · XeF4 Lewis Structure, Molecular Geometry, Hybridization, and
PH3 · XeF4 Lewis Structure, Molecular Geometry
PH4 · XeF4 Geometry and Hybridization
PH5 · Lewis Dot Structure of XeF4 (Xenon TetraFluoride)
PH6 · Hybridization of xenon tetrafluoride, its bond angle, properties, types
PH7 · Hybridization of Xenon Tetrafluoride
PH8 · Hybridization of XeF4: Hybridization of Xe in Xenon Tetrafluoride
PH9 · Hybridization of XeF4: Hybridization of Xe in Xenon Tetrafluoride
PH10 · Hybridization of XeF4 (Xenon Tetrafluoride)
PH11 · Hybridisation of xenon tetrafluoride using Group theory
PH12 · 6.3: Xenon Tetrafluoride